Introduction
The lesson plans presented here were developed by high school and community college instructors of STEM subjects. All were participants in a Research Experiences for Teachers (RET) program at the University of South Florida, for which the focus was functional materials. Owing to the challenge of matching a particular research project to a lesson that can be applied in a particular classroom, not all of the plans have a strong Materials Science focus. However, most of them do – which provides the opportunity to place them within context by supplying here some background information to the subject of Materials Science and Engineering. Along the way, we will provide links to many of the lesson plans and also to short reports on specific materials that were prepared by the RET participants.
Let's begin with an overall description of the field. Materials Science seeks to relate composition, microscopic structure and processing of materials to their properties. For instance, why do some materials conduct electricity and others not? Why does the addition of carbon to iron create a new metal with more desirable properties? And why is it possible to stress a material beyond its elastic limit and increase its strength in the process?
Materials Engineering goes one step further and includes a consideration of the end use of the material. Materials Engineers must use these relationships between properties and material composition, structure, and processing to select (or create) materials that will work in a particular application, whether it is for a stronger building, a lighter airplane, or a processing vessel that will withstand a highly corrosive acid.
So what are functional materials? Three different definitions are provided to the right =>
The last definition is particularly helpful. For most of the history of mankind, materials science and engineering focused on creating materials that could be used for construction, transportation, clothing and utensils. Properties of interest included strength, heat resistance, electrical conductivity, durability, density, and malleability. Functional materials are materials that have additional properties that allow them to be used in additional, non-traditional applications – including piezoelectric materials used in microphones, biocompatible polymers with specific functionalities that allow them to bond with living tissue, superconductors that allow magnetic levitation, and zeolites that contain cages that allow for the separation of a gas mixture into individual components. Part of the excitement of studying functional materials is dreaming up ways that a recently discovered material property can be exploited to create a new application!
Although it is not a perfect classification scheme, materials are usually assigned to one of three different classes: Metals, polymers and ceramics. A representative of each class is shown in the image of a hip prosthesis (Figure 1) at the top of this page. Below, we will provide a little background for each class of material.
Metals
A metal might be a single element (such as gold) or a mixture of elements, such as solder (a mixture of lead and tin). Metals tend to conduct electricity and heat very well and can be shaped or drawn into wire. These properties arise from the way that the atoms in metals are bonded together.
In a solid metal, atoms tend to arrange themselves in a regular repeating structure called a lattice. There are a number of ways that the atoms may arrange themselves – leading to different crystal structures. Most metals form one of three crystal structures – face centered cubic (FCC), body centered cubic (BCC) and hexagonal close packed (HCP). Figure 3 shows unit cells (the smallest repeating unit in a lattice) for the BCC and FCC crystal structures. Note that each unit cell is a cube with an atom at each corner. The FCC structure has, in addition, at atom in the center of each face while the BCC structure has an atom in the center of the cell.
Other materials besides metals can have similar crystal structures. What sets metals apart is how the atoms are bonded together in the lattice. You have probably studied ionic bonding, in which electrons are taken from one atom and added to another, and covalent bonding, in which electrons are shared between atoms. In metals, a different type of bond, called the metallic bond, exists. In the metallic bond (see Figure 4), the valence electrons of the individual atoms are delocalized (not bound to a specific nucleus) and form a sea of negatively charged mobile electrons surrounding the positively charged nuclei. It is this characteristic that gives metals their unique properties.
Although metal atoms in the solid state are arranged in a lattice, the lattice does not typically extend through the entire macroscopic sample of a material. In fact, it is generally very difficult to make such a material (known as a single crystal). Instead, most metals are polycrystalline, meaning they are composed of many smaller grains of single crystals. An image of a polycrystalline metal, showing the individual grains, is shown in Figure 5. Grain size is directly tied to strength and can be controlled during processing.
As mentioned above, a metal can be a mixture of two or more different elements. Examples are solder (mentioned earlier), brass (a mixture of copper and zinc) and steel (a mixture of carbon and iron). Such combinations are called alloys, and often have properties that are more desirable than those of the corresponding pure elements. For instance, copper-nickel alloy of about 60% nickel has a tensile strength higher than that of pure nickel or of pure copper.
Metals are usually not found pure in nature. Most are in the form of ores such as hematite (Fe2O3), bauxite (Al2O3) and Galena (PbS). To be useful, the metals have to be extracted from the ores using a process called smelting – which is heating in the presence of either oxygen or carbon (depending on the ore). Smelting is highly energy intensive – which is why it is far more preferable to obtain metals by recycling when possible.
Metals have a number of functional properties. For some examples, click here to learn more about cobalt and its use in near-field communications. At the microscopic level, metal nanoparticles have applications to photo-catalysis. Click the links to read short papers on silver nanoparticles and metal nanoparticles in general.
Several of the lesson plans relate to metals including one in which students synthesize and test their own catalyst, another in which students synthesize and analyze metal nanoparticles and a third in which students learn about graphical interpretation of data using transmission electron microscopy and ultra-violet/visible light spectroscopy images from measurements on silver nanocubes.
Polymers
Polymers are materials comprised of long chain molecules formed by reacting smaller units with each other. They may occur naturally (rubber and cellulose) or they may be synthesized (polystyrene or poly vinyl chloride). Development of man-made polymers began in the 19th century but really took off during and following World War II and the polymer industry today rivals the metals industry in size.
Polymers are made from small molecules called monomers. In the example shown in Figure 7, the monomer is ethylene and the polymer is polyethylene. The repeat unit in the polymer chain consists of the two carbon atoms in ethylene plus their associated hydrogen atoms. The molecular weight of ethylene is 28 but it is not uncommon for polyethylene to have a molecular weight in the millions – so each chain of polyethylene contains a very large number of monomer repeat units. Reacting the monomers together to form a polymer is called polymerization and can accomplished with several different methods. Two common ones are chain polymerization and condensation polymerization.
Chains with only one type of monomer are known as homopolymers. If two or more different types of monomers are involved, the resulting polymer is called a copolymer. Like metal alloys, the properties of a copolymer are often more desirable than those of either of the constituent homopolymers. Copolymers can have several configurations or arrangements of the monomers along the chain as shown in Figure 8. In random copolymers the arrangement of the two different repeat units is, as you've probably guessed, random. In block copolymers, the majority of repeat units are bonded with repeat units of the same type. Only occasionally does one repeat unit react with a different type, which leads to the repeat units appearing in blocks. In an alternating copolymer, two repeat units only bond with each other, as a result of a specific reaction that only occurs between groups on unlike monomers. Branched copolymers have chains of one repeat unit branching off of a chain of the other one.
Thermoplastics are polymers that can soften under heating, thus allowing them to be reshaped and recycled. This is possible because, while there are fairly strong covalent bonds within the polymer chains, there are no bonds between chains – only comparatively weaker van der Waals forces. Examples of thermoplastics polyethylene and polystyrene.
Thermosets, on the other hand, do not soften upon heating and therefore cannot easily be recycled. In thermosets, there is crosslinking (via covalent bonds) between the chains and the polymer is essentially a three dimensional network as shown in Figure 9. Examples of thermosets include vulcanized rubber (used in car tires) and epoxies.
Click the links here to read about specific polymers including Nylon, polyisobutylene, poly-N-iso propylacrylamide (which has biomedical applications), CYTOP (used in creating non-wetting surfaces), elastin-like polypeptides or ELPs (used in tissue engineering), siloxane and polystyrene.
A number of lesson plans here involve polymers including one in which a hydrogel is used as a cell culture platform, another that illustrates selective diffusion through polymer membranes, one in which students create their own polystyrene fibers, and a lesson in which students study how wetting of a surface is affected by the coating material on the surface.
Ceramics
Ceramics is the category that anything that is not a metal or polymer tends to get shoved into. However, a large fraction of ceramics have the following in common: They are comprised of atoms of both metals and nonmetals but their overall properties are non-metallic in nature. Examples include silicate glass, silicon nitride, titanium dioxide and clays like Kaolin.
Bonds in most ceramics are ionic or covalent. The valence electrons are not delocalized as they are in metals leading to stronger bonds in ceramics. As a result, ceramics tend to be strong, but brittle, and do not conduct heat or electricity at ordinary conditions. They are used as electrical insulators, as thermal insulation and in applications where corrosion protection is required.
The crystal structures of ceramics are more varied than for metals (see structure for SiO2 in Figure 11) and are dictated primarily by two things: The relative number of cations (positive ions) and anions (negative ions) and their relative sizes. The first of these is dictated by the anionic and cationic charges. For example, NaCl has the same number of Na+ as Cl- ions. CaF2 has twice as many F- ions as Ca2+ ions. These two parameters greatly influence how the cations and anions can pack together and thus what crystal structure results.
Certain ceramics also exhibit interesting properties which make them useful as functional materials. One such property is piezoelectricity. If a piezoelectric material (see Figure 12) is stressed, an electric field is established – meaning it can transform a mechanical signal into an electrical one. The reverse is also true – meaning the material can be deformed by putting an electric field across it. Relatively few ceramics have this property but they find use in many applications including air bags, pressure sensors, phonograph needles, microphones and sound sensors. Click the link for a lesson plan in which students build a circuit to store energy generated by deforming a piezoelectric crystal.
Zeolites (also known as molecular sieves) are crystalline materials which belong to the class of aluminosilicates. They have a three-dimensional pore system with pores of precise diameter, as shown in Figure 13. These pore structures act like cages for small molecules that come into contact with them and, because of this property, zeolites find applications in removal of impurities, separation processes, catalysis and hydrogen storage.
Somewhat related to zeolites are metal-organic frameworks or MOFs. MOFs are materials in which metal clusters are connected to organic ligands to form a variety of structures. Many of these are three dimensional and have the same property of large pore spaces as zeolites. As a result, they have many of the same applications. Click the links to read more about metal organic frameworks in general, to learn about a particular MOF (MOF 505), or to learn about a special type of MOF called a metal–metalloporphyrin framework (MMPF).
Finally, we will note that some lesson plans cross over between material classes. Students will learn more about both conductors and insulators if they follow the lesson on building a capacitor. And one participant developed an instructional unit that provides an overview of the entire field of Material Science through teaching students about states of matter.
References and Image Sources:
[1] www3.imperial.ac.uk/materials/research/functionalmaterials
[2] www.npl.co.uk/science-technology/functional-materials/
[3] www.matuk.co.uk/docs/Functioanmat.pdf
[4] https://commons.wikimedia.org/wiki/File:Hip_prosthesis.jpg
[5] https://commons.wikimedia.org/wiki/File:Gallium_crystals.jpg
[6] https://upload.wikimedia.org/wikipedia/commons/thumb/a/aa/
IronAlfa%26IronGamma.svg/320px-IronAlfa%26IronGamma.svg.png)
[7] https://commons.wikimedia.org/wiki/File:Metallic_bond_Zn.svg
[8] https://commons.wikimedia.org/wiki/File:CrystalGrain.jpg
[9] http://www.publicdomainpictures.net/view-image.php?image=112756&picture=plastic-bottles
[10] https://www.flickr.com/photos/102642344@N02/15907823547
[11] https://commons.wikimedia.org/wiki/File:Overview_Copolymers.svg
[12] https://commons.wikimedia.org/wiki/
File:Structures_of_macromolecules.png
[13] https://commons.wikimedia.org/wiki/
File:Bridge_from_dental_porcelain.jpg
[14] https://commons.wikimedia.org/wiki/File:Beta-quartz-CM-2D-balls.png
[15] https://commons.wikimedia.org/wiki/File:SchemaPiezo.gif
[16] https://commons.wikimedia.org/wiki/File:Zeolite-ZSM-5-vdW.png
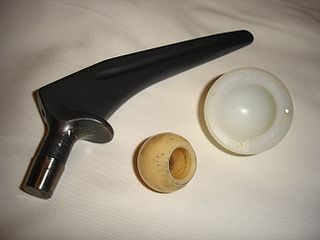
Figure 1. A titanium hip prosthesis, with a ceramic head and polyethylene acetabular cup (Photo by Nuno Nogueira, [4])
Functional Materials
Functional materials possess particular native properties and functions of their own. For example, ferroelectricity, piezoelectricity, magnetism or energy storage functions. [1]
Functional materials exploit coupling between multiple variables - for example, transforming mechanical energy to electrical energy in piezoelectric materials, or providing electrical control of magnetic properties. [2]
Functional materials perform specific functions other than possessing a load bearing capacity. Examples include semiconductors, magnetic materials, piezoelectrics and ionic conductors. [3]

Figure 2. Crystals of the metal Gallium [5]
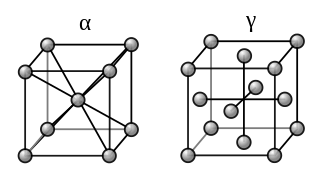
Figure 3. Unit cells for the Body Centered Cubic (left) and Face Centered Cubic (right) crystal structures. [6]
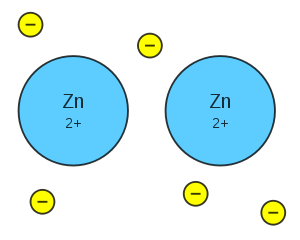
Figure 4. Illustration of a metallic bond in zinc with delocalized electrons shown in yellow and nuclei in blue [7]

Figure 5. Grain boundaries in a titanium alloy (photo by Edward Pleshakov, [8])

Figure 6. Bottles made from a synthetic polymer [9]

Figure 7. Polyethylene (polymer) structure (right) and ethylene (monomer) structure (left) (Image by Zappys Technological Solutions [10])
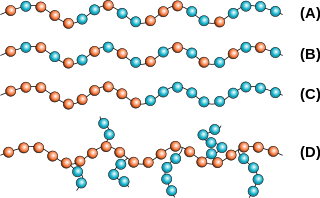
Figure 8. Copolymer structures: (A) Random, (B)Alternating, (C) Block and (D) Branched [11]

Figure 9. A thermoplastic polymer (left) and a crosslinked thermoset polymer (right) [12]
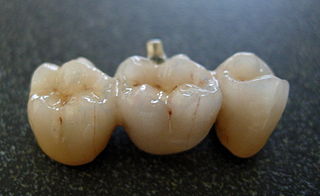
Figure 10. A ceramic dental bridge [13]
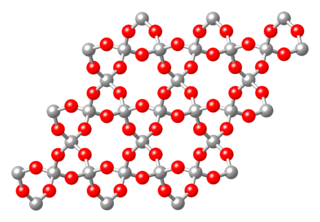
Figure 11. Crystal structure of quartz (SiO2) [14]

Figure 12. The piezoelectric effect (Image by Tizeff, [15])
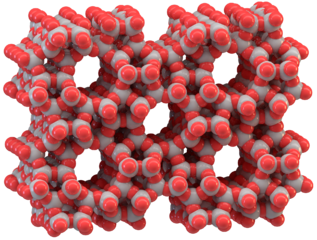
Figure 13. The structure of zeolite, ZSM-5 (Image by Thomas Splettstoesser, [16])